 M.I.C.C.
Manchester Ice Cloud Chamber
M.I.C.C.
Manchester Ice Cloud Chamber
 Site and content created using Xara Designer Pro 6
Site and content created using Xara Designer Pro 6
 Ice Crystal Aggregation
If ice crystals are allowed to grow for a sufficient amount of time, they will start to coalesce and form chains
(Fig. 1). These chains of crystals are known as aggregates and have optical properties that are different to
individual ice crystals. In particular, the anvils of equatorial thunderstorms have been shown to contain
aggregates of horizontally aligned plates which act like mirrors, reflecting a lot of incoming radiation (which is
intense at the equator and thus important to
understand) (Liou, 1973; Ackermann et al. , 1988;
Stephens et al., 1990; Hartmann et al., 1992;
McFarquhar and Heymsfield, 1997). The
reflection of solar radiation back into space by
such ice clouds can have a significant cooling
effect on the planet, countering the warming
effect of greenhouse gases. This is discussed in
more detail in the light scattering section. The
reflective properties of the ice clouds containing a
substantial aggregate presence can be better
understood by learning about the properties of
aggregates formed under a range of conditions.
Aggregation is also an important process to
consider in numerical models of cloud and
precipitation processes.
There are a number of microphysical mechanisms conducive to the formation of aggregates. The adhesive
properties of an ice crystal’s surface are related to its temperature. At higher temperatures closer to 0°C, the
ice surfaces are ‘stickier’ increasing the chance of cohesion of two colliding ice crystals. As temperature
reduces, the surfaces become colder and less sticky, and this makes aggregation less likely from this
adhesive effect (Fig. 2). Once two ice crystals have collided together, the process of sintering can act to bond
the particles together. Sintering is the process whereby water molecules diffuse from a place of lower
curvature (elsewhere on the surface) or the environment to a place of higher curvature (e.g. the boundary
where two crystals meet) (Fig. 2). Another method by which ice crystals can stick together relies on the
interlocking of different parts of their structure. For example, the multi-branching arms of dendritic crystals can
more easily interlock if they collide than the relatively smooth faces of columnar or plate crystals. This process
is therefore dependent on habit and thus temperature and humidity values conducive to such shape formation
(Fig. 2). Experimental work has shown that at -12°C (and in general between -10 and -15°C), there is an
increased occurrence of aggregation attributed to this effect (Hobbs et al. 1974). One final aggregation
mechanism relates to the coalescence of ice crystals in the presence of high electric fields—such as those
found in thunderstorms. Research has shown that while such fields do not increase the attraction of crystals
together from distances, it does allow crystals to remain connected on collision for long enough to allow the
sintering process to ‘glue’ them together. This electrical effect is independent of temperature unlike the
adhesive effect associated with the surface properties of ice, as discussed earlier (Latham and Saunders,
1970a,b) (Fig. 2). Greater numbers of crystals present in a cloud also increase the probability of aggregation
between them; more crystals will lead to an increasing chance of any two of them colliding.
In the laboratory studies, we aim to quantify ice crystal aggregation as a function of cloud temperature. The
physical properties of the ice crystals can be measured using the Cloud Particle Imager (CPI) and help identify
their sticking and collision efficiencies. Aggregates form readily in the 10 m high cloud chamber. Clouds of
supercooled water droplets are grown and nucleated at the top (turned into ice crystals), and the substantial
vertical height of the chamber allows sufficient growth time for crystal coalescence to occur and the formation
of chain aggregates. The aggregates formed are then measured by the CPI as they fall to the base of the
chamber, and their properties such as length and structure as a function of the ambient temperature can be
measured. Some preliminary results are shown in Fig. 3 in which chain aggregates of different habits have
been grown to about 1 mm in length. In nature, the largest aggregates of ice crystals are formed during heavy
snow events, when ice crystals can aggregate into clumps which are several cms in size (Fig. 4).
References
Ackermann, T. P., Liou, K. N., Valero, F. P. J., and Pfister, L., 1988, Heating rates in tropical anvils., J. Atmos. Sci., 45, 1606-1623.
Hartman, D. L., Ockert-Bell, M. E., and Michelsen, M. L., 1992, The effect of cloud type on the earth’s energy balance: Global analysis., J.
Climate, 5, 1281–1304.
Hobbs, P., S. Chang, and J. Locatelli, 1974: The dimension and aggregation of ice crystals in natural clouds. J. Geophys. Res., 79,2199–2206.
Latham, J., Saunders, C. P. R., 1970a, Experimental measurements of the collection efficiencies of ice crystals in electric fields. Q. J. R.
Meteorol. Soc., 96,257–265.
Latham, J., Saunders, C. P. R., 1970b, The electrostatic forces on charged ice crystals separated by small distances in an electric field. Q. J.
R. Meteorol. Soc., 96,266–272
Liou, K. N., 1973, Transfer of solar irradiance through cirrus cloud layers., J. Geophys. Res., 78, 1409–1418.
McFarquhar, G. M., and Heymsfield, A. J., 1997, Parameterization of tropical cirrus ice crystal size distributions and implications for radiative
transfer: Results from cepex., J. Atmos. Sci., 54, 2187–2200.
Saunders, C. P. R. and Wahab, N. M. A., 1975, The influence of electric fields on the aggregation of ice crystals, J. Meteorol. Soc. Jpn., 53,
121–126.
Stephens, G. L., Tsay, S., Stackhouse, P. W., and Flatau, P. J., 1990, The relevance of the microphysical and radiative properties of cirrus
clouds to climate and climatic feedback., J. Atmos. Sci., 47, 1742–1753.
Wahab, N. M. A., 1974, ‘Ice crystal interactions in electric fields’, PhD thesis, UMIST.
Ice Crystal Aggregation
If ice crystals are allowed to grow for a sufficient amount of time, they will start to coalesce and form chains
(Fig. 1). These chains of crystals are known as aggregates and have optical properties that are different to
individual ice crystals. In particular, the anvils of equatorial thunderstorms have been shown to contain
aggregates of horizontally aligned plates which act like mirrors, reflecting a lot of incoming radiation (which is
intense at the equator and thus important to
understand) (Liou, 1973; Ackermann et al. , 1988;
Stephens et al., 1990; Hartmann et al., 1992;
McFarquhar and Heymsfield, 1997). The
reflection of solar radiation back into space by
such ice clouds can have a significant cooling
effect on the planet, countering the warming
effect of greenhouse gases. This is discussed in
more detail in the light scattering section. The
reflective properties of the ice clouds containing a
substantial aggregate presence can be better
understood by learning about the properties of
aggregates formed under a range of conditions.
Aggregation is also an important process to
consider in numerical models of cloud and
precipitation processes.
There are a number of microphysical mechanisms conducive to the formation of aggregates. The adhesive
properties of an ice crystal’s surface are related to its temperature. At higher temperatures closer to 0°C, the
ice surfaces are ‘stickier’ increasing the chance of cohesion of two colliding ice crystals. As temperature
reduces, the surfaces become colder and less sticky, and this makes aggregation less likely from this
adhesive effect (Fig. 2). Once two ice crystals have collided together, the process of sintering can act to bond
the particles together. Sintering is the process whereby water molecules diffuse from a place of lower
curvature (elsewhere on the surface) or the environment to a place of higher curvature (e.g. the boundary
where two crystals meet) (Fig. 2). Another method by which ice crystals can stick together relies on the
interlocking of different parts of their structure. For example, the multi-branching arms of dendritic crystals can
more easily interlock if they collide than the relatively smooth faces of columnar or plate crystals. This process
is therefore dependent on habit and thus temperature and humidity values conducive to such shape formation
(Fig. 2). Experimental work has shown that at -12°C (and in general between -10 and -15°C), there is an
increased occurrence of aggregation attributed to this effect (Hobbs et al. 1974). One final aggregation
mechanism relates to the coalescence of ice crystals in the presence of high electric fields—such as those
found in thunderstorms. Research has shown that while such fields do not increase the attraction of crystals
together from distances, it does allow crystals to remain connected on collision for long enough to allow the
sintering process to ‘glue’ them together. This electrical effect is independent of temperature unlike the
adhesive effect associated with the surface properties of ice, as discussed earlier (Latham and Saunders,
1970a,b) (Fig. 2). Greater numbers of crystals present in a cloud also increase the probability of aggregation
between them; more crystals will lead to an increasing chance of any two of them colliding.
In the laboratory studies, we aim to quantify ice crystal aggregation as a function of cloud temperature. The
physical properties of the ice crystals can be measured using the Cloud Particle Imager (CPI) and help identify
their sticking and collision efficiencies. Aggregates form readily in the 10 m high cloud chamber. Clouds of
supercooled water droplets are grown and nucleated at the top (turned into ice crystals), and the substantial
vertical height of the chamber allows sufficient growth time for crystal coalescence to occur and the formation
of chain aggregates. The aggregates formed are then measured by the CPI as they fall to the base of the
chamber, and their properties such as length and structure as a function of the ambient temperature can be
measured. Some preliminary results are shown in Fig. 3 in which chain aggregates of different habits have
been grown to about 1 mm in length. In nature, the largest aggregates of ice crystals are formed during heavy
snow events, when ice crystals can aggregate into clumps which are several cms in size (Fig. 4).
References
Ackermann, T. P., Liou, K. N., Valero, F. P. J., and Pfister, L., 1988, Heating rates in tropical anvils., J. Atmos. Sci., 45, 1606-1623.
Hartman, D. L., Ockert-Bell, M. E., and Michelsen, M. L., 1992, The effect of cloud type on the earth’s energy balance: Global analysis., J.
Climate, 5, 1281–1304.
Hobbs, P., S. Chang, and J. Locatelli, 1974: The dimension and aggregation of ice crystals in natural clouds. J. Geophys. Res., 79,2199–2206.
Latham, J., Saunders, C. P. R., 1970a, Experimental measurements of the collection efficiencies of ice crystals in electric fields. Q. J. R.
Meteorol. Soc., 96,257–265.
Latham, J., Saunders, C. P. R., 1970b, The electrostatic forces on charged ice crystals separated by small distances in an electric field. Q. J.
R. Meteorol. Soc., 96,266–272
Liou, K. N., 1973, Transfer of solar irradiance through cirrus cloud layers., J. Geophys. Res., 78, 1409–1418.
McFarquhar, G. M., and Heymsfield, A. J., 1997, Parameterization of tropical cirrus ice crystal size distributions and implications for radiative
transfer: Results from cepex., J. Atmos. Sci., 54, 2187–2200.
Saunders, C. P. R. and Wahab, N. M. A., 1975, The influence of electric fields on the aggregation of ice crystals, J. Meteorol. Soc. Jpn., 53,
121–126.
Stephens, G. L., Tsay, S., Stackhouse, P. W., and Flatau, P. J., 1990, The relevance of the microphysical and radiative properties of cirrus
clouds to climate and climatic feedback., J. Atmos. Sci., 47, 1742–1753.
Wahab, N. M. A., 1974, ‘Ice crystal interactions in electric fields’, PhD thesis, UMIST.

 Fig. 1. Chains of ice crystal aggregates consisting of 30–50 µm
plate crystals. Adapted from Wahab (1974), and Saunders and
Wahab (1975).
Fig. 1. Chains of ice crystal aggregates consisting of 30–50 µm
plate crystals. Adapted from Wahab (1974), and Saunders and
Wahab (1975).

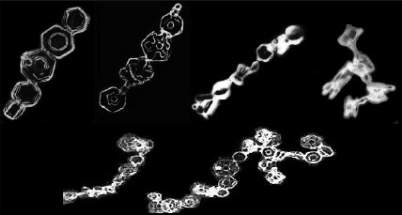
 Fig. 2. An illustration of the different microphysical ice crystal aggregation processes. The adhesive effect of a crystal’s ice
surface is temperature dependent, with higher temperatures being more conducive to crystal-sticking. Sintering is the process
by which vapour growth at the contact point of two crystals aids in cementing the crystals together once touching. Interlocking
involves a physical connection of parts of two colliding crystals. High electric fields can also help keep ice crystals together for
long enough for sintering to form a bond, and this is independent of temperature.
Fig. 2. An illustration of the different microphysical ice crystal aggregation processes. The adhesive effect of a crystal’s ice
surface is temperature dependent, with higher temperatures being more conducive to crystal-sticking. Sintering is the process
by which vapour growth at the contact point of two crystals aids in cementing the crystals together once touching. Interlocking
involves a physical connection of parts of two colliding crystals. High electric fields can also help keep ice crystals together for
long enough for sintering to form a bond, and this is independent of temperature.
 Plates
Plates
 Dendrites
Dendrites
 Sectored plates
Sectored plates
 Long columns
Long columns
 Short columns
Short columns
 Fig. 4. Large aggregate snowflakes 10,000-50,000 µm (1-5 cm) in diameter falling in Manchester City Centre,
England. Recorded at approximately 10:30 am on 22nd December 2009. These aggregates, having fallen
hundreds of meters through the lower atmosphere, are substantially bigger than can be created in our 10 m
tall fall tube. Snow accumulations of several cm resulted and the snow was good quality, i.e. 'wet' and sticky (good for
snowball/man making)!
Note: Unfortunately, YouTube compresses even 1080p videos tremendously, and for fast moving images containing small
objects, this compression substantially reduces the quality of these fine details. The frame rate is also significantly less
than the original uploaded video.
Fig. 4. Large aggregate snowflakes 10,000-50,000 µm (1-5 cm) in diameter falling in Manchester City Centre,
England. Recorded at approximately 10:30 am on 22nd December 2009. These aggregates, having fallen
hundreds of meters through the lower atmosphere, are substantially bigger than can be created in our 10 m
tall fall tube. Snow accumulations of several cm resulted and the snow was good quality, i.e. 'wet' and sticky (good for
snowball/man making)!
Note: Unfortunately, YouTube compresses even 1080p videos tremendously, and for fast moving images containing small
objects, this compression substantially reduces the quality of these fine details. The frame rate is also significantly less
than the original uploaded video.




 Choose
resolution
Choose
resolution




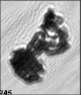




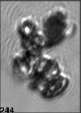

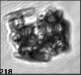
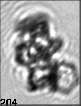
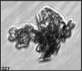




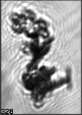
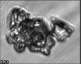
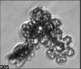
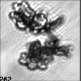

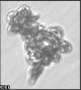

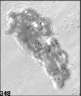
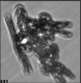
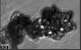
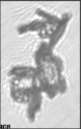






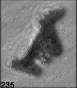




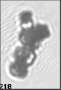
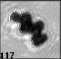

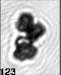 Fig. 3. Example images of some of the aggregate chains formed recently at various temperatures using MICC. Click the image
thumbnails to enlarge. Multiplying the small number in the bottom left of each image by 2.4 gives the physical dimension in
micrometers.
Fig. 3. Example images of some of the aggregate chains formed recently at various temperatures using MICC. Click the image
thumbnails to enlarge. Multiplying the small number in the bottom left of each image by 2.4 gives the physical dimension in
micrometers.
Website technical facts
Software Xara Designer Pro 6 was used to create this
website and all it’s content, including graphics,
panoramic photos and Flash animations. Creation
involved a new approach to web design: graphical,
WYSIWYG object placement without the need to code
a single line of HTML or JavaScript.
The site is highly optimised: the entire 19 page
website consumes a grand total of just 4.5 MB,
including all html, Flash, photo and graphic image files
(plus there's a separate 12.6 MB of panoramic photo
data—eight 50 million pixel photos). All graphics and
photos are screen optimised for the web, and all this
makes the site as fast as possible to load.
 M.I.C.C.
Manchester Ice Cloud Chamber
M.I.C.C.
Manchester Ice Cloud Chamber
 Site and content created using Xara Designer Pro 6
Site and content created using Xara Designer Pro 6
 Ice Crystal Aggregation
If ice crystals are allowed to grow for a sufficient amount of time, they will start to coalesce and form chains
(Fig. 1). These chains of crystals are known as aggregates and have optical properties that are different to
individual ice crystals. In particular, the anvils of equatorial thunderstorms have been shown to contain
aggregates of horizontally aligned plates which act like mirrors, reflecting a lot of incoming radiation (which is
intense at the equator and thus important to
understand) (Liou, 1973; Ackermann et al. , 1988;
Stephens et al., 1990; Hartmann et al., 1992;
McFarquhar and Heymsfield, 1997). The
reflection of solar radiation back into space by
such ice clouds can have a significant cooling
effect on the planet, countering the warming
effect of greenhouse gases. This is discussed in
more detail in the light scattering section. The
reflective properties of the ice clouds containing a
substantial aggregate presence can be better
understood by learning about the properties of
aggregates formed under a range of conditions.
Aggregation is also an important process to
consider in numerical models of cloud and
precipitation processes.
There are a number of microphysical mechanisms conducive to the formation of aggregates. The adhesive
properties of an ice crystal’s surface are related to its temperature. At higher temperatures closer to 0°C, the
ice surfaces are ‘stickier’ increasing the chance of cohesion of two colliding ice crystals. As temperature
reduces, the surfaces become colder and less sticky, and this makes aggregation less likely from this
adhesive effect (Fig. 2). Once two ice crystals have collided together, the process of sintering can act to bond
the particles together. Sintering is the process whereby water molecules diffuse from a place of lower
curvature (elsewhere on the surface) or the environment to a place of higher curvature (e.g. the boundary
where two crystals meet) (Fig. 2). Another method by which ice crystals can stick together relies on the
interlocking of different parts of their structure. For example, the multi-branching arms of dendritic crystals can
more easily interlock if they collide than the relatively smooth faces of columnar or plate crystals. This process
is therefore dependent on habit and thus temperature and humidity values conducive to such shape formation
(Fig. 2). Experimental work has shown that at -12°C (and in general between -10 and -15°C), there is an
increased occurrence of aggregation attributed to this effect (Hobbs et al. 1974). One final aggregation
mechanism relates to the coalescence of ice crystals in the presence of high electric fields—such as those
found in thunderstorms. Research has shown that while such fields do not increase the attraction of crystals
together from distances, it does allow crystals to remain connected on collision for long enough to allow the
sintering process to ‘glue’ them together. This electrical effect is independent of temperature unlike the
adhesive effect associated with the surface properties of ice, as discussed earlier (Latham and Saunders,
1970a,b) (Fig. 2). Greater numbers of crystals present in a cloud also increase the probability of aggregation
between them; more crystals will lead to an increasing chance of any two of them colliding.
In the laboratory studies, we aim to quantify ice crystal aggregation as a function of cloud temperature. The
physical properties of the ice crystals can be measured using the Cloud Particle Imager (CPI) and help identify
their sticking and collision efficiencies. Aggregates form readily in the 10 m high cloud chamber. Clouds of
supercooled water droplets are grown and nucleated at the top (turned into ice crystals), and the substantial
vertical height of the chamber allows sufficient growth time for crystal coalescence to occur and the formation
of chain aggregates. The aggregates formed are then measured by the CPI as they fall to the base of the
chamber, and their properties such as length and structure as a function of the ambient temperature can be
measured. Some preliminary results are shown in Fig. 3 in which chain aggregates of different habits have
been grown to about 1 mm in length. In nature, the largest aggregates of ice crystals are formed during heavy
snow events, when ice crystals can aggregate into clumps which are several cms in size (Fig. 4).
References
Ackermann, T. P., Liou, K. N., Valero, F. P. J., and Pfister, L., 1988, Heating rates in tropical anvils., J. Atmos. Sci., 45, 1606-1623.
Hartman, D. L., Ockert-Bell, M. E., and Michelsen, M. L., 1992, The effect of cloud type on the earth’s energy balance: Global analysis., J.
Climate, 5, 1281–1304.
Hobbs, P., S. Chang, and J. Locatelli, 1974: The dimension and aggregation of ice crystals in natural clouds. J. Geophys. Res., 79,2199–2206.
Latham, J., Saunders, C. P. R., 1970a, Experimental measurements of the collection efficiencies of ice crystals in electric fields. Q. J. R.
Meteorol. Soc., 96,257–265.
Latham, J., Saunders, C. P. R., 1970b, The electrostatic forces on charged ice crystals separated by small distances in an electric field. Q. J.
R. Meteorol. Soc., 96,266–272
Liou, K. N., 1973, Transfer of solar irradiance through cirrus cloud layers., J. Geophys. Res., 78, 1409–1418.
McFarquhar, G. M., and Heymsfield, A. J., 1997, Parameterization of tropical cirrus ice crystal size distributions and implications for radiative
transfer: Results from cepex., J. Atmos. Sci., 54, 2187–2200.
Saunders, C. P. R. and Wahab, N. M. A., 1975, The influence of electric fields on the aggregation of ice crystals, J. Meteorol. Soc. Jpn., 53,
121–126.
Stephens, G. L., Tsay, S., Stackhouse, P. W., and Flatau, P. J., 1990, The relevance of the microphysical and radiative properties of cirrus
clouds to climate and climatic feedback., J. Atmos. Sci., 47, 1742–1753.
Wahab, N. M. A., 1974, ‘Ice crystal interactions in electric fields’, PhD thesis, UMIST.
Ice Crystal Aggregation
If ice crystals are allowed to grow for a sufficient amount of time, they will start to coalesce and form chains
(Fig. 1). These chains of crystals are known as aggregates and have optical properties that are different to
individual ice crystals. In particular, the anvils of equatorial thunderstorms have been shown to contain
aggregates of horizontally aligned plates which act like mirrors, reflecting a lot of incoming radiation (which is
intense at the equator and thus important to
understand) (Liou, 1973; Ackermann et al. , 1988;
Stephens et al., 1990; Hartmann et al., 1992;
McFarquhar and Heymsfield, 1997). The
reflection of solar radiation back into space by
such ice clouds can have a significant cooling
effect on the planet, countering the warming
effect of greenhouse gases. This is discussed in
more detail in the light scattering section. The
reflective properties of the ice clouds containing a
substantial aggregate presence can be better
understood by learning about the properties of
aggregates formed under a range of conditions.
Aggregation is also an important process to
consider in numerical models of cloud and
precipitation processes.
There are a number of microphysical mechanisms conducive to the formation of aggregates. The adhesive
properties of an ice crystal’s surface are related to its temperature. At higher temperatures closer to 0°C, the
ice surfaces are ‘stickier’ increasing the chance of cohesion of two colliding ice crystals. As temperature
reduces, the surfaces become colder and less sticky, and this makes aggregation less likely from this
adhesive effect (Fig. 2). Once two ice crystals have collided together, the process of sintering can act to bond
the particles together. Sintering is the process whereby water molecules diffuse from a place of lower
curvature (elsewhere on the surface) or the environment to a place of higher curvature (e.g. the boundary
where two crystals meet) (Fig. 2). Another method by which ice crystals can stick together relies on the
interlocking of different parts of their structure. For example, the multi-branching arms of dendritic crystals can
more easily interlock if they collide than the relatively smooth faces of columnar or plate crystals. This process
is therefore dependent on habit and thus temperature and humidity values conducive to such shape formation
(Fig. 2). Experimental work has shown that at -12°C (and in general between -10 and -15°C), there is an
increased occurrence of aggregation attributed to this effect (Hobbs et al. 1974). One final aggregation
mechanism relates to the coalescence of ice crystals in the presence of high electric fields—such as those
found in thunderstorms. Research has shown that while such fields do not increase the attraction of crystals
together from distances, it does allow crystals to remain connected on collision for long enough to allow the
sintering process to ‘glue’ them together. This electrical effect is independent of temperature unlike the
adhesive effect associated with the surface properties of ice, as discussed earlier (Latham and Saunders,
1970a,b) (Fig. 2). Greater numbers of crystals present in a cloud also increase the probability of aggregation
between them; more crystals will lead to an increasing chance of any two of them colliding.
In the laboratory studies, we aim to quantify ice crystal aggregation as a function of cloud temperature. The
physical properties of the ice crystals can be measured using the Cloud Particle Imager (CPI) and help identify
their sticking and collision efficiencies. Aggregates form readily in the 10 m high cloud chamber. Clouds of
supercooled water droplets are grown and nucleated at the top (turned into ice crystals), and the substantial
vertical height of the chamber allows sufficient growth time for crystal coalescence to occur and the formation
of chain aggregates. The aggregates formed are then measured by the CPI as they fall to the base of the
chamber, and their properties such as length and structure as a function of the ambient temperature can be
measured. Some preliminary results are shown in Fig. 3 in which chain aggregates of different habits have
been grown to about 1 mm in length. In nature, the largest aggregates of ice crystals are formed during heavy
snow events, when ice crystals can aggregate into clumps which are several cms in size (Fig. 4).
References
Ackermann, T. P., Liou, K. N., Valero, F. P. J., and Pfister, L., 1988, Heating rates in tropical anvils., J. Atmos. Sci., 45, 1606-1623.
Hartman, D. L., Ockert-Bell, M. E., and Michelsen, M. L., 1992, The effect of cloud type on the earth’s energy balance: Global analysis., J.
Climate, 5, 1281–1304.
Hobbs, P., S. Chang, and J. Locatelli, 1974: The dimension and aggregation of ice crystals in natural clouds. J. Geophys. Res., 79,2199–2206.
Latham, J., Saunders, C. P. R., 1970a, Experimental measurements of the collection efficiencies of ice crystals in electric fields. Q. J. R.
Meteorol. Soc., 96,257–265.
Latham, J., Saunders, C. P. R., 1970b, The electrostatic forces on charged ice crystals separated by small distances in an electric field. Q. J.
R. Meteorol. Soc., 96,266–272
Liou, K. N., 1973, Transfer of solar irradiance through cirrus cloud layers., J. Geophys. Res., 78, 1409–1418.
McFarquhar, G. M., and Heymsfield, A. J., 1997, Parameterization of tropical cirrus ice crystal size distributions and implications for radiative
transfer: Results from cepex., J. Atmos. Sci., 54, 2187–2200.
Saunders, C. P. R. and Wahab, N. M. A., 1975, The influence of electric fields on the aggregation of ice crystals, J. Meteorol. Soc. Jpn., 53,
121–126.
Stephens, G. L., Tsay, S., Stackhouse, P. W., and Flatau, P. J., 1990, The relevance of the microphysical and radiative properties of cirrus
clouds to climate and climatic feedback., J. Atmos. Sci., 47, 1742–1753.
Wahab, N. M. A., 1974, ‘Ice crystal interactions in electric fields’, PhD thesis, UMIST.

 Fig. 1. Chains of ice crystal aggregates consisting of 30–50 µm
plate crystals. Adapted from Wahab (1974), and Saunders and
Wahab (1975).
Fig. 1. Chains of ice crystal aggregates consisting of 30–50 µm
plate crystals. Adapted from Wahab (1974), and Saunders and
Wahab (1975).


 Fig. 2. An illustration of the different microphysical ice crystal aggregation processes. The adhesive effect of a crystal’s ice
surface is temperature dependent, with higher temperatures being more conducive to crystal-sticking. Sintering is the process
by which vapour growth at the contact point of two crystals aids in cementing the crystals together once touching. Interlocking
involves a physical connection of parts of two colliding crystals. High electric fields can also help keep ice crystals together for
long enough for sintering to form a bond, and this is independent of temperature.
Fig. 2. An illustration of the different microphysical ice crystal aggregation processes. The adhesive effect of a crystal’s ice
surface is temperature dependent, with higher temperatures being more conducive to crystal-sticking. Sintering is the process
by which vapour growth at the contact point of two crystals aids in cementing the crystals together once touching. Interlocking
involves a physical connection of parts of two colliding crystals. High electric fields can also help keep ice crystals together for
long enough for sintering to form a bond, and this is independent of temperature.

 Plates
Plates
 Dendrites
Dendrites
 Sectored plates
Sectored plates
 Long columns
Long columns
 Short columns
Short columns





 Choose
resolution
Choose
resolution











































 Fig. 3. Example images of some of the aggregate chains formed recently at various temperatures using MICC. Click the image
thumbnails to enlarge. Multiplying the small number in the bottom left of each image by 2.4 gives the physical dimension in
micrometers.
Fig. 3. Example images of some of the aggregate chains formed recently at various temperatures using MICC. Click the image
thumbnails to enlarge. Multiplying the small number in the bottom left of each image by 2.4 gives the physical dimension in
micrometers.